- Article
- Source: Campus Sanofi
- 23 Oct 2023
Treatment of CAT Clexane (enoxaparin)


Clexane is indicated in adults for the extended treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE) and prevention of its recurrence in patients with active cancer based on the RIETECAT trial results.
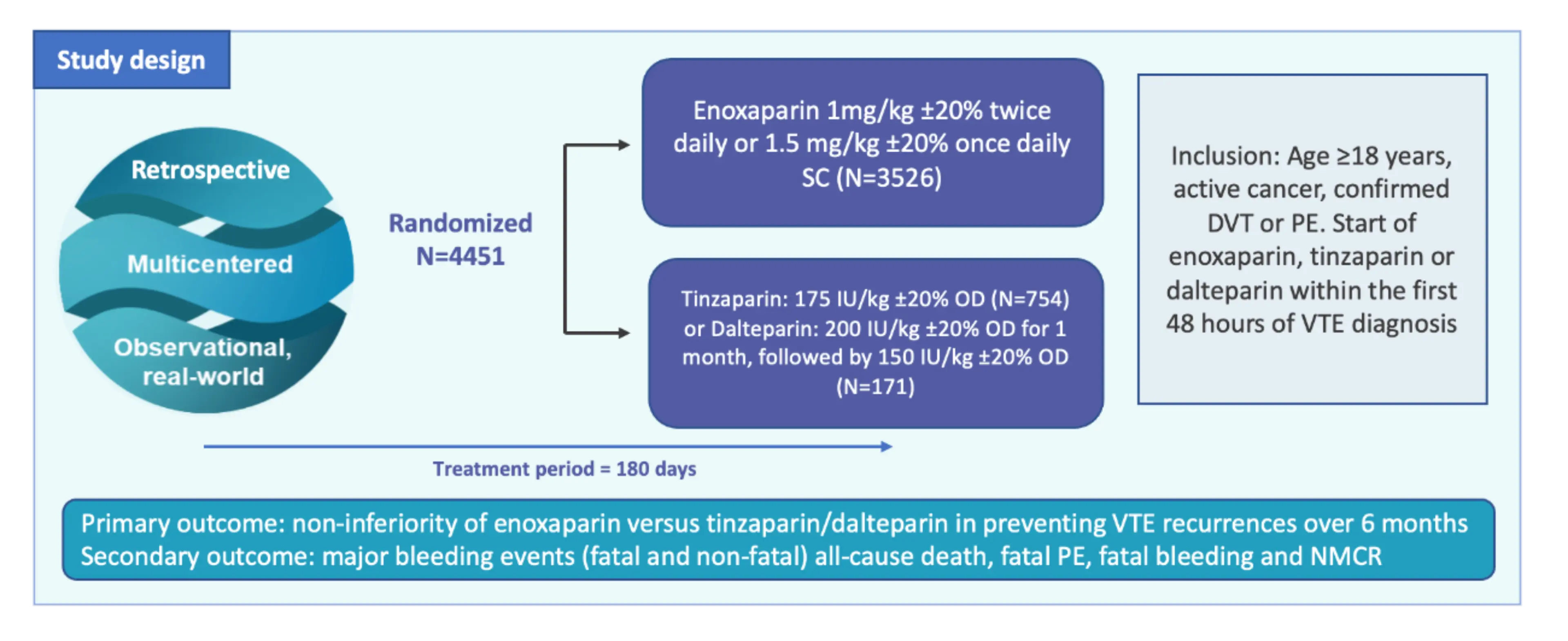
RIETECAT trial is a multinational observational retrospective cohort study using data from the RIETE registry.1
It is the first and largest study comparing the effectiveness and safety of enoxaparin against dalterparin and tinzaparin in a real-world cohort of cancer patients with VTE.1
RIETECAT outcomes
Cancer patients with VTE receiving full dose enoxaparin or tinzaparin/dalteparin had comparable effectiveness and safety outcomes over a 6 month period.
OR: 0.79 (95%CI. 049-1.28) p=0.004
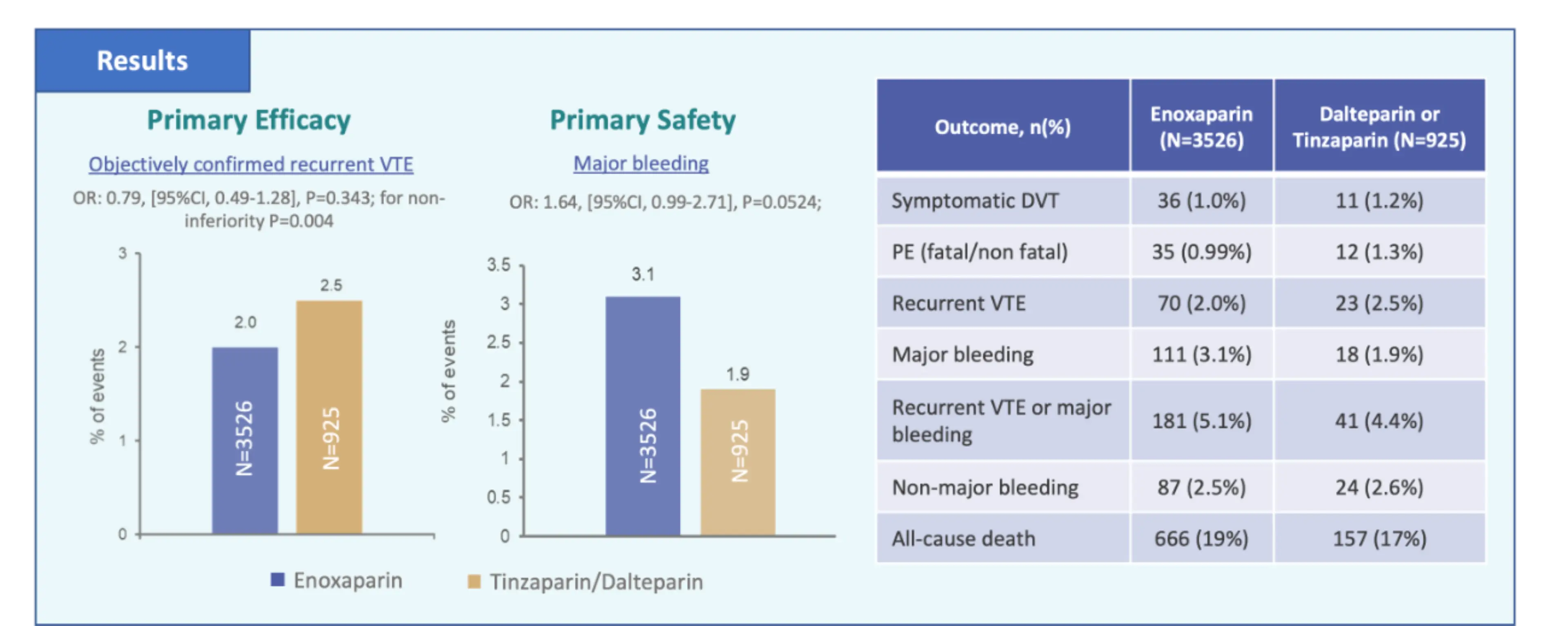
The efficacy outcome was a composite of symptomatic DVT and fatal or non-fatal PE and the safety outcome was a composite of major bleeding events (fatal or non fatal) and non major bleeds of clinical significance.
Due to the non-interventional nature of the study, comparability of patients between groups may be difficult to achieve; however multivariate Cox model was used to minimize the confounding bias.
Patients treated with enoxaparin had a non-significantly lower risk for VTE recurrences, a non-significantly higher risk for major bleeding, and similar risks for non-major bleeding or death over 6 months compared to tinzaparin/dalteparin.
Footnote
CAT=cancer-associated thrombosis; VTE=Venous Thromboembolism; CI=confidence interval; OR=Odds Ratio; SC=subcutaneous; IU=international unit; OD=Once daily; PE pulmonary embolism; NMCR=Non-major clinically relevant.
.jpg)
References
- Trujillo-Sanos J et al. Res Pract Thromb Haemost. 2022;6:e12736.
MAT-XU-2204401 (v3.0) Date of Preparation: October 2023